20 Which of the Following Is True About Chemical Energy
2 products have lower stability than reactants. If the potential energy in the chemical bonds of the reactants is greater than the potential energy in the chemical bonds of the product A energy must be supplied for the reaction to occur.

Types Of Energy Article Khan Academy
Chemical energy cannot be converted into mechanical energy.

. If the anvil is dropped that potential energy transforms to kinetic energy as the anvil moves faster and faster toward Earth. C the chemical reaction equalizes the potential energy levels. Which of the following is not true about energy in ecosystems.
D energy has not been gained or lost. Combustion reaction converts chemical energy into light and heat. Mechanical energy is the sum of an objects kinetic and potential energy.
Digested to convert chemical energy into other forms of energy used by cells. Energy is transferred between organisms. Energy is recycled after flowing through ecosystems.
What is true of chemical energy is that chemical energy can be stored more efficiently as glucose than it can be as ATP. The reaction is exergonic C. A Since electrons atoms molecules and ions are moving they possess kinetic energy.
The correct answer is therefore A. Chemical energy is stored in the bonds between the atoms in compounds. Energy spent on cellular respiration is available to higher trophic levels.
1 reactants have high energy compared to products. B energy is released by the reaction. Most energy enters ecosystems as sunlight.
Energy is created through conduction. Combustion reaction converts chemical energy into light and heat. Energy changes from one form to another.
What mass of Epsom salt will dissolve in 500 mL of 20C water. Chemical energy is released when bonds in adp are broken to form atp. If the image does not load see Figure 3 in the exam 5 images PDF in the exam 5 folder on Husky CT Products б K8əua Free Reactants A Course of reaction - O A.
4 energy is released into the surroundings. Energy is broken in more ways as only ADP - D is also wrong. Ecosystems require a continuous influx of new energy.
All the other answers in this case dont really apply. Autotrophs typically capture about 90 of the available energy from the sun through photosynthesis. E energy is not a factor in the reaction.
B Energy is being converted between potential energy and kinetic energy as particles within chemicals bump into each other. The following chemical equation represent two different chemical reactions. Burned to produce heat and light.
1 point carbon dioxide water oxygen. 3 products are more stable than reactants. Two forces slide together that are touching.
Chemical energy is stored in the bonds of 1 point oxygen molecules. One of the following is not a characteristic of an endothermic reaction. Which of the following is true about chemical energy.
Energy used in the production pf offspring is available to higher trophic levels. 2 products have lower stability than reactants. The first statement is true.
Even though a lot of energy is converted into non-mechanical forms all of the energy is accounted for. All of the chemical energy in the gasoline is converted into the mechanical energy of the vehicle. Chemical bonds MIGHT NOT be always broken by energy absorption.
Chemical energy can only be stored as adp. Radiant energy is also called. Splitting water into hydrogen and oxygen.
When ATP is broken down to ADP we get a release of energy - B and C are therefore wrong. Chemical energy is absorbed in a reaction. There are reactions which require energy for activation and reaction proceeds with the evolution of energy.
The reaction is spontaneous OB. 1 Which of the following is a chemical change. A chemical reaction takes place.
Which is a starting material for cellular respiration. This stored energy is transformed when bonds are broken or formed through chemical reactions. 1 point Boiling of water.
Chemical energy is stored when bonds in atp are broken to form adp. Melting a cube of ice. C The energy released by chemical reactions is due.
2 What is the mass of water that results from combining 20g of hydrogen with. The amount of energy depends on the nature of reactants. Moreover there are a horde of examples where energy absorbing reactions occur.
Energy transformations occur when. QUESTION 20 Which of the following is true regarding the chemical reaction depicted in this graph. Chemical energy can be stored more efficiently as glucose than it can be as atp.
In a trophic pyramid biomass represents chemical energy. A form of energy you use when cheering for your favorite team. Dissolving 10 grams of salt in water.

Revision Resources Science Doctor Energy Facts Physics Paper Energy Transfer
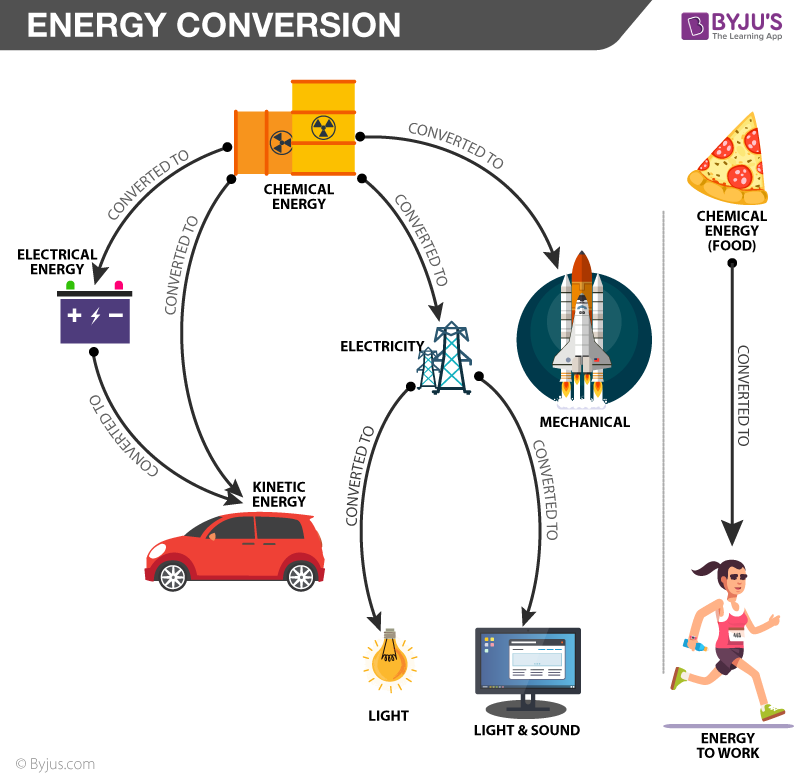
Energy Conversion Law Of Energy Conversion With Examples

Researchers Produce Two Biofuels From A Single Algae Jet Fuel Hydroponics Fuel

Iec Unit 4 In 2021 Words Interactive The Unit

Endothermic Exothermic Reactions 1 Exothermic Reaction Chemical Energy Reactions

Chemical And Physical Changes Chemical And Physical Changes Physical Change Physics

Energy Warm Ups Bell Ringers Science Teaching Resources Middle School Science Teacher Middle School Science Activities

Chemistry Inquiry Prototype Design Project Pbl Dragon S Den Shark Tank Chemical Reactions High School Science Chemistry

Omg Beautiful Fantastic Revision Notes Revision Notes Chemistry Notes

Pulp Paper Industry Paper Industry Process Control Technology Solutions

Hsc Chemistry Board Question Papers 2020 Chemistry Paper Question Paper Chemistry

25 Inspired Photo Of Sight Word Coloring Pages Entitlementtrap Com What Is Energy Reading Worksheets Reading Comprehension Worksheets
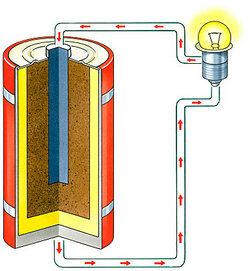
Examples Of Chemical Energy In Everyday Life

Powerowl High Capacity Lr44 Batteries 40 Pack Ag13 357 303 Sr44 Premium Alkaline Battery 1 5v Button Coin Cell Batteries In 2022 Alkaline Battery Energy Density Lr44 Battery

Pin By Ashleigh Kruse On Balancing Equations Chemical Equation Balancing Equations Physical Science

What Is Chemical Energy Definition Examples Applications Of Chemical Energy
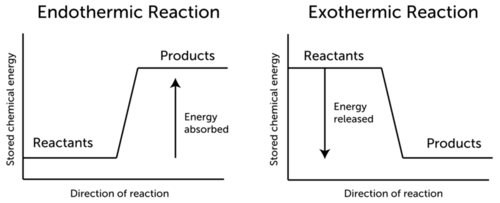
Conservation Of Energy In Chemical Reactions Ck 12 Foundation


Comments
Post a Comment